The shortfall of monkeypox vaccine doses in the USA, anticipated to final for months, is elevating pressing questions on how effectively and for a way lengthy a single shot could defend towards the virus.
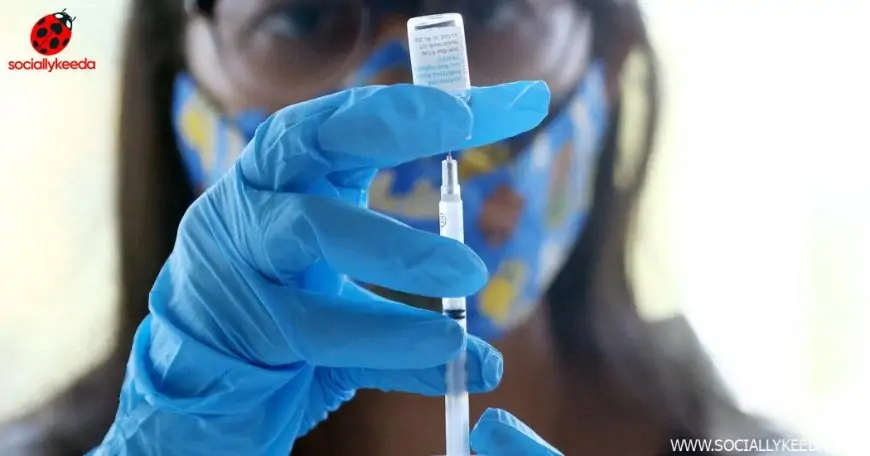
The vaccine, known as Jynneos, is accepted as a two-dose routine, however most individuals vulnerable to an infection have been receiving one dose — if they will discover it. Now the scarcity has led federal officers to contemplate a hardly ever used method: a so-called dose-sparing technique, which provides pictures that every include simply one-fifth of a single dose.
For many recipients, one shot needs to be sufficient to beat back severe sickness, and there may be some proof that even smaller doses may be efficient. However preliminary analysis suggests that individuals with H.I.V. or different circumstances that weaken the immune system could also be much less protected than individuals who don’t have such diseases, in accordance with some specialists.
“One dose is better than none,” mentioned Dr. Alexandra Yonts, an infectious ailments doctor at Kids’s Nationwide Hospital in Washington, D.C. However individuals with “H.I.V. and other immunocompromised individuals need to be aware that they may not have an adequate level of protection from infection, even with vaccination,” she added.
Even two weeks after the shot, when the antibody response is underway, immunocompromised individuals nonetheless could have to “use all other precautions to prevent being exposed, per public health guidance,” she mentioned.
The findings additionally counsel that some males needs to be prioritized for full vaccination. Given the provision constraints, which may be tough.
Federal officers have ordered almost seven million doses of Jynneos, however the pictures is not going to arrive for months. Up to now, the Biden administration has shipped about 600,000 doses to states. It mentioned final week that 800,000 further doses have been being allotted to states, however the distribution may take weeks.
Confronted with shortages, some cities, together with Washington and New York, are proscribing second doses to stretch their provides. Officers on the Meals and Drug Administration and the C.D.C. have disagreed with that technique, noting that Jynneos is accepted as a vaccine to be given in two doses spaced 28 days aside.
However as federal health officers declared a public health emergency on Thursday, Dr. Robert Califf, the commissioner of the F.D.A., mentioned the company was now contemplating authorizing pictures that include simply one-fifth of the common dose, delivered between layers of the pores and skin as a substitute of below it.
What to Know Concerning the Monkeypox Virus
What's monkeypox? Monkeypox is a virus much like smallpox, however signs are much less extreme. It was found in 1958, after outbreaks occurred in monkeys stored for analysis. The virus was primarily present in components of Central and West Africa, however in latest weeks it has unfold to dozens of nations and contaminated tens of 1000's of individuals, overwhelmingly males who've intercourse with males. On July 23, the World Health Group declared monkeypox a world health emergency.
The F.D.A. would wish to grant Jynneos an emergency use authorization to ensure that it to be administered this fashion.
The dose-sparing method has been used when provides of different vaccines are scarce. However giving intradermal pictures requires more talent than is required for more conventional immunizations.
One shot might be sufficient to forestall extreme signs in most individuals, and the dose-sparing technique may go simply as effectively. But it surely’s unclear whether or not a scaled-back routine is sufficient to forestall an infection, and in that case, how lengthy that immunity could final, federal health officers mentioned.
“We’re in a data-free zone,” mentioned Dr. Emily Erbelding, an infectious ailments knowledgeable on the Nationwide Institutes of Health, who oversaw testing of Covid vaccines in particular populations.
One oft-cited statistic says that the vaccine is 85 p.c efficient towards monkeypox. That knowledge derives not from trials of Jynneos, however from a small 1988 research that seemed on the incidence of monkeypox amongst individuals who had been inoculated for smallpox earlier of their lives.
No massive medical trial of Jynneos as a monkeypox vaccine was carried out in people earlier than its approval. As a substitute, the F.D.A. relied on measures of antibody responses in small teams of individuals after immunization with Jynneos in contrast with these produced by ACAM2000, an earlier vaccine for smallpox.
In research led by its producer, Bavarian Nordic, two doses of Jynneos yielded antibody ranges in people that have been about the identical as these after one shot of ACAM2000.
Antibody ranges after the primary shot of Jynneos initially rose for 2 weeks after which remained flat till the second dose 4 weeks later, after they soared to very excessive ranges — increased than these recorded with ACAM2000.
Scientists read that to imply if the primary dose shouldn't be adopted by a second, the safety will not be long-lasting.
“Ideally, a second dose would be administered if protection for more than that four-week period is desired,” mentioned Dr. Yonts, who reviewed the info for the F.D.A. as a workers scientist.
She added that delaying the second dose to eight weeks could be affordable. “But if it’s going to be like six months, then I think that prioritization would lean more toward those that are more severely immunocompromised,” she mentioned.
Injecting one-fifth of a daily dose of Jynneos between pores and skin layers, because the F.D.A. urged on Thursday, could also be efficient, in accordance with restricted analysis. The pores and skin has many more of the immune cells that reply to vaccines.
However the analysis could be very restricted. Scientists on the N.I.H. had deliberate to check the dose-sparing technique in a medical trial set to start in just a few weeks. It's unclear whether or not these plans can be shelved or sped up.
Details about how Jynneos performs in individuals with H.I.V., significantly in these with extreme immune issues, was already scant. In a single research carried out by Bavarian Nordic, antibody response to vaccination tended to be diminished: At 28 days after the primary shot, 67 p.c of these with H.I.V. produced antibodies, in contrast with 84 p.c of uninfected individuals.
Whereas Dr. Yonts mentioned the info from that trial was not conclusive, decreased antibody response is usually seen amongst immunocompromised individuals given different vaccines. Whereas evaluating Covid vaccines, for instance, researchers discovered that sufferers with H.I.V. have been more prone to have breakthrough infections.
“Individuals with severe or moderate immune suppression are recommended for additional doses of common vaccines,” mentioned Keri Althoff, an epidemiologist on the Johns Hopkins Bloomberg College of Public Health, who led the Covid vaccine research. “As immune suppression increases, the response to the vaccines does decrease.”
The C.D.C. and the New York Metropolis Division of Health say Jynneos is protected for individuals with H.I.V., however the businesses haven't addressed its effectiveness in that inhabitants.
In contrast, health officers in Britain say that for individuals who “are H.I.V. positive or have any other condition or treatment leading to a weakened immune system, the vaccine may not protect you as well.”
The vaccine’s bundle insert additionally notes that immunocompromised individuals “may have a diminished immune response.”
“Two shots may be very important in this population, which is something that is not actually happening in the public health response,” mentioned Dr. Chloe Orkin, an infectious illness doctor at Queen Mary College of London, referring to immunocompromised individuals.
However till more doses can be found, state and native health departments could not have a lot of a selection apart from to stay with scaled-back regimens.
“In an environment of scarcity, we have to do everything we can to get the benefits of vaccine to the city as quickly as possible,” mentioned Patrick Gallahue, a spokesman for New York Metropolis’s health division, in a press release.
Keep Tuned with Sociallykeeda.com for more Health news.
Denial of duty! SociallyKeeda.com is an automated aggregator across the international media. All of the content material can be found free on Web. We've got simply organized it in a single platform for instructional function solely. In every content material, the hyperlink to the first supply is specified. All logos belong to their rightful house owners, all supplies to their authors. If you're the proprietor of the content material and don't want us to publish your supplies on our web site, please contact us by electronic mail – [email protected]. The content material can be deleted inside 24 hours.
| ???? Follow US On Google News | Click on Right here |
| ???? Fb Web page | Click on Right here |
| ???? Telegram Channel | Click on Right here |
| Click on Right here | |
| ???? Filmy Publish | Click on Right here |
| ???? Web site | Click on Right here |



![[WATCH VIDEO] Sophie Rain and sister Sierra Rain as Black Spiderman goes viral [WATCH VIDEO] Sophie Rain and sister Sierra Rain as Black Spiderman goes viral](https://www.sociallykeeda.com/uploads/images/202403/image_140x98_660976c59cce0.webp)






![[FULL WATCH VIDEO] Will Levis And Gia Duddy Leak Video Viral On Social Media [FULL WATCH VIDEO] Will Levis And Gia Duddy Leak Video Viral On Social Media](https://www.sociallykeeda.com/uploads/images/202405/image_140x98_6651e7ae8038d.webp)

