- Commercial-
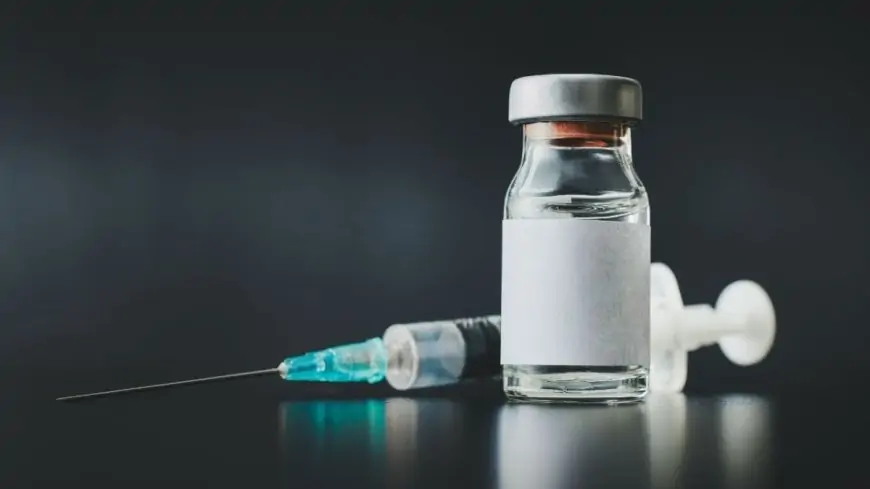
In all international locations of the world, the provision of the corona vaccine stays a significant trigger for concern. In the meantime, there are reviews that round 1.5 crore doses have been affected as a consequence of a glitch within the manufacturing of the Corona vaccine by pharma firm Johnson & Johnson. Bloomberg has been knowledgeable about this by 2 individuals concerned in vaccine manufacturing. Nonetheless, the corporate has stated that due to this, the provision of the vaccine won't be affected.
Defect in a batch of vaccine
Based on the report, one and a half million doses of Johnson & Johnson vaccine are reported to be unhealthy in a US plant. In an announcement issued by Johnson & Johnson, one batch of the vaccine failed within the high quality check.
Forma firm officers discovered a batch of vaccines on the Baltimore plant operated by Emergent Biosolutions, which didn't meet the standard check and didn't meet high quality requirements.
The information of the issue within the manufacture of vaccine within the plant was revealed by the New York Instances on Wednesday. Within the manufacturing plant, there was a multitude within the manufacturing of the vaccine as a result of fault of the employees.
The corporate will keep and broaden the plant
The has given this data to the US FDA. On the similar time, the US FDA has confirmed that they're conscious of this whole growth. As a result of high quality and safety are his largest precedence.
On the similar time, Johnson & Johnson goes to extend the variety of officers within the emergency facility plant. Specialists shall be deployed at each website for manufacturing, technical and high quality operation within the plant, in order that vital directions and help in vaccine manufacturing may be offered.
After this incident got here to mild, the corporate has stated that it'll not have an effect on the provision of the vaccine. They're dedicated to supply of the Corona vaccine on time.

![[Review] The Communal Place at Joo Chiat introduces new Miso-centric dishes [Review] The Communal Place at Joo Chiat introduces new Miso-centric dishes](https://www.sociallykeeda.com/uploads/images/202312/image_430x256_656f5943053ec.webp)
![[WATCH VIDEO] Sophie Rain and sister Sierra Rain as Black Spiderman goes viral [WATCH VIDEO] Sophie Rain and sister Sierra Rain as Black Spiderman goes viral](https://www.sociallykeeda.com/uploads/images/202403/image_140x98_660976c59cce0.webp)





![[FULL WATCH VIDEO] Will Levis And Gia Duddy Leak Video Viral On Social Media [FULL WATCH VIDEO] Will Levis And Gia Duddy Leak Video Viral On Social Media](https://www.sociallykeeda.com/uploads/images/202405/image_140x98_6651e7ae8038d.webp)


